


The Future
of Itch Relief
Ideal First-Line Treatment
Numelvi™ (atinvicitinib tablets for dogs) is a second-generation Janus kinase (JAK) inhibitor. It is indicated for the treatment of pruritus associated with allergic dermatitis including atopic dermatitis in dogs.

The first and only veterinary JAK inhibitor that is highly selective for JAK1.*

The only JAK inhibitor safe for use in dogs and puppies as young as 6 months of age.

Relief starts within 2-4 hours.1

Easy once-daily dosing from day 1.
* Over the other JAK enzymes in in vitro assays.
Selectivity in Action
Watch how Numelvi selectively targets the primary driver of itch and inflammation.
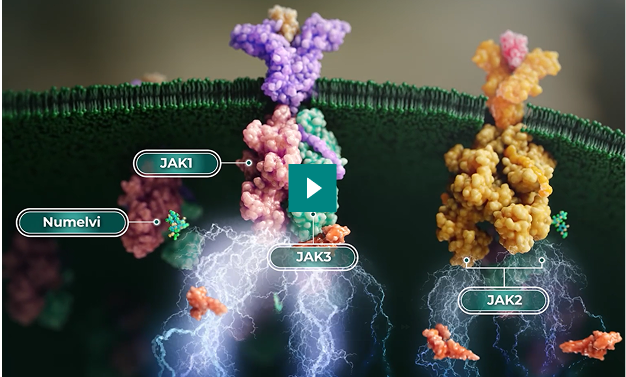
The Science of Selectivity

Cytokines use JAK enzymes to stimulate immune cells.

JAK1 is the primary driver of itch and inflammation.

First-generation JAK inhibitors target the JAK enzymes with little to no selectivity.2, 1

High selectivity for JAK1 helps minimize interference with the beneficial functions of other JAKs.

Numelvi is at least 10x more selective for JAK1 over the other JAKs.†
First-generation JAK inhibitors are not selective (< 2x).
† In in vitro assays.
Proven Safe in Dog Studies4
Numelvi is safe in dogs — even in puppies as young as 6 months of age.

Interactions
- No interactions with routine treatments
- Adequate serological response to vaccination during treatment
Few, mild treatment-related adverse effects
- Most commonly mild diarrhea and vomiting, sometimes associated with lethargy and decreased appetite, that did not require treatment
- No clinically relevant changes in red blood cell parameters, clinical chemistry, or urinalysis5
- Serious infections, papillomas, and skin lumps were not reported
Reduction of allergy-mediated inflammation
- Leads to a reduction of inflammation associated white blood cell counts (within reference range)
Hear From the Experts
Find out what veterinarians are saying about Numelvi.

Dr. Christine McKinney, DACVD, Veterinary Dermatologist, MSD Animal Health

Dr. Sam Geller, Veterinary general practitioner, USA

Dr. Bob Sarsfield, Veterinary general practitioner, USA
Compare Numelvi With the Competition
* Over the other JAK enzymes in in vitro assays.
Have a question about Numelvi?
Relief Starts Within 2-4 Hours1
Once-daily dosing starts relieving itch from day 1 with continued improvement thereafter7
>81% of dogs showed substantial reduction in itch within one week7§
‡ When compared to oclacitinib
§ A ≥2 cm reduction in owner pruritus visual analog scale score (PVAS) is clinically relevant8
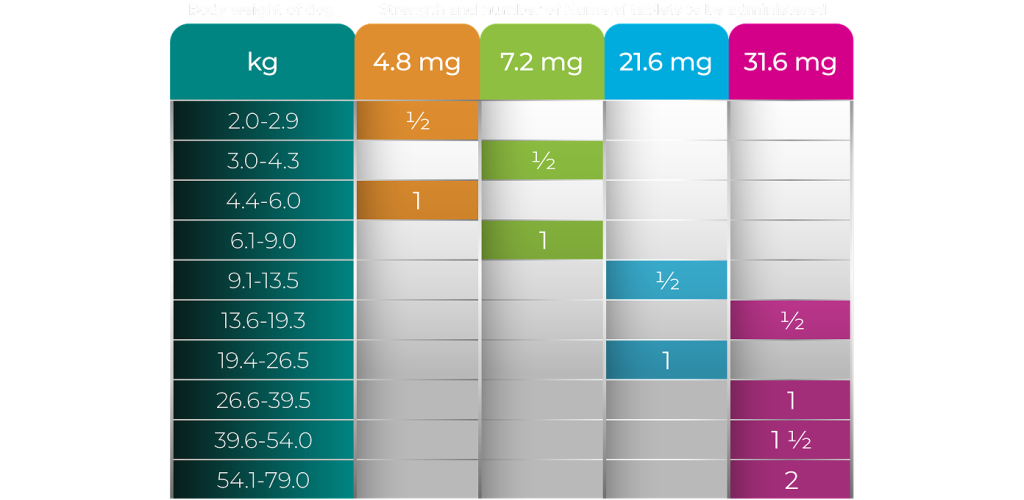
Available in 4 Tablet Strengths for Simple Dosing

Easy Dosing With Most Dogs Requiring 1 Whole or One-half Tablet
The average recommended dose is 1.0 mg of atinvicitinib per kg body weight orally once daily at or around mealtime.
Simplicity and Convenience
Numelvi makes managing itch seamless.
Simple for Veterinarians
- Safe option for all dogs and puppies from 6 months of age
- Easy dosing helps owner compliance
- Ready-to-dispense packs
Simple for Dog Owners
- Once-daily dosing from the start
- Consistent relief with consistent dosing
- Reassuring safety
Have a question about Numelvi?
NUMELVI tablets for dogs. In the absence of available data, NUMELVI should not be used in dogs less than 6 months old. It is recommended to investigate and treat complicating factors, such as bacterial, fungal or parasitic infections (e.g., flea, Demodex mites), as well as any underlying causes (e.g., flea allergy, contact allergy, food allergy) of allergic and atopic dermatitis. Do not use in cases of hypersensitivity to the active substance or to any of the excipients. The safety of NUMELVI has not been established during pregnancy and lactation or in breeding dogs. Therefore, the use in those dogs is not recommended. For complete information, refer to the Summary of Product Characteristics.
References
1. Study Summary REF-11201. MSD Animal Health. 2. Gonzales AJ, Bowman JW, Fici GJ, et al. J Vet Pharmacol Ther. 2014;37(4):317-324. 3. European Medicines Agency. Zenrelia: PUAR – Product Information. 2025. 4. European Medicines Agency. Numelvi: PUAR – Product Information. 2025. 5. Data on file. Study Summary REF-11202. MSD Animal Health. 6. European Medicines Agency. Apoquel: PUAR – Product Information & Assessment Report. 2025. 7. Data on file. Study Summary REF-11194. MSD Animal Health. 8. Cosgrove SB, Wren JA, Cleaver DM, et al. Vet Dermatol. 2013;24(5):479-e114.
There’s More to Learn About the Future of Itch Relief
Please fill out the form below to request a call from an MSD Animal Health representative.
